Gestion des amendements calciques et basiques :Calcium cycle and interests
Calcium plays a crucial role in soil structure and plant resistance to diseases and pests, as well as root growth. These combined effects contribute to better agricultural productivity. It is essential to understand its various aspects to optimize amendment management.
Organic effluents, such as poultry droppings and manure, are important sources of calcium for soils. Their strategic use can improve soil fertility and the growth of crops.
Acid tests can help evaluate calcium activity in the soil, providing valuable information to adjust fertilization practices and improve nutrient availability for plants.
Understanding the differences between calcium and limestone in the soil is essential for effective amendment management, as these elements have different impacts on soil structure and plant health.
Cet article fait partie d'une formation AgroLeague en 4 parties :
- Gestion des amendements calciques et basiques partie 1 : le pH, fonctionnement et impact
- Gestion des amendements calciques et basiques partie 2 : cycle et intérêts du calcium
- Gestion des amendements calciques et basiques partie 3 : quels éléments pour la prise de décision ?
- Gestion des amendements calciques et basiques partie 4 : stratégies d'apports
The calcium cycle and types of calcium inputs
Distinguishing calcium and limestone
Limestone is a calcium reserve, but calcium itself has no effect on soil pH. In limestone (CaCO3), it is the base that affects pH, not the calcium. Calcium is important for plant nutrition and soil structuring.
Active limestone is the portion of limestone that can rapidly solubilize calcium. A high limestone content active can cause iron chlorosis because the strong solubilization of Ca2+ ions can block iron uptake. Having no active limestone in your soil is not a problem. It simply means that little calcium will be solubilized. Calcium can also have an effect of phosphorus insolubilization.

The calcium cycle
Carbonate quarries with lime kilns heat carbonates at high temperatures to obtain quicklime, constituting basic mineral amendments.
There are also naturally occurring carbonates in soils. Weathering of the parent rock is a source of mineral calcium that exchanges more or less rapidly in the soil.
Soluble calcium in the soil allows to fill the Cation Exchange Capacity (CEC) to have a greater retention capacity. It serves to feed plants, which are grazed by animals or exported at harvest. Livestock manure and crop residues return calcium. Additionally, some is lost by runoff and leaching.

Some types of calcium-containing inputs
Mineral products
- Gypsum: CaSO42-
- Calcium carbonate, marl, quarry sands: CaCO3
- Dolomite: CaMgCO3
- Quicklime: CaO
- Hydrated lime: CaOH
Organic products
The table below summarizes the compositions found in various livestock effluents:

In descending order of calcium content, we find: Poultry droppings > poultry manure > Compost bovine manure > bovine manure > Slurry. Regarding pH, effluents are generally basic.
The different roles of calcium
Effect on soil
- Structuring effect: Calcium forms calcium bridges between organic matter and clays to form the clay-humus complex and create good soil aeration, and more broadly, better structuring compared to soils with low calcium content and low pH.
- Retention potential of mineral elements: Mineral elements are stored on the clay-humus complex, which can retain elements more or less well depending on the cation exchange capacity (CEC). CEC is the maximum total amount of cations a soil can trap at a given pH. It is influenced by pH and the amount of cations available in the soil. The higher the CEC, the greater the soil's retention potential for elements.
You can test soil reaction before applying calcium inputs by spreading a bag of gypsum over an area of 100 m2, i.e., a 10 m x 10 m square. The next two images illustrate this test with the first photo showing the plot with an input equivalent to 3 t/ha of gypsum, and the second the control plot. A real difference in structuring is observed: the structure is more crumbly on the surface, more aerated.
Effect on plants
- Calcium is important at the beginning of the cycle for the formation of their cell walls. It allows better mechanical resistance to bio-aggressors, especially piercing-sucking insects.
- It ensures good root growth.
- It is involved in enzyme production, particularly nitrate reductase which transforms nitrates into nitrites.
Most sensitive plants
Potato
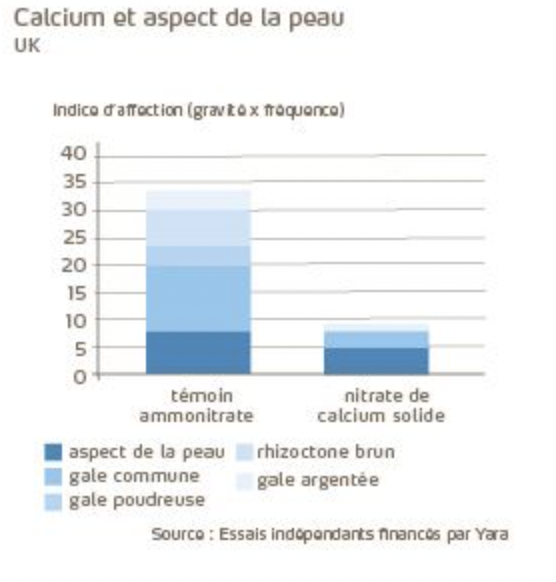
The trial results below illustrate the benefit of calcium on the skin appearance of the potato. Lower proportions of silver scurf are observed in the treatment with calcium inputs.
Alfalfa
Very demanding in potassium, alfalfa is also a crop with high Ca needs: it exports about 30 kg CaO/T of dry matter. Assuming a hypothetical production of 10 t/ha, exports are 300 U/ha of CaO.

How to position your approach?
For meadows and crops, liming buffers soil acidification linked to nitrogen spreading and biological activity. It stimulates biological activity and improves soil structure.
The acid test
A hydrochloric acid test allows to visually identify the active limestone content in the soil based on the reaction obtained. This test can be performed at 2 depths (5 and 30 cm) to highlight possible limestone leaching, but also on casts to highlight limestone rising by earthworms to the surface.
The principle is to apply 10% diluted hydrochloric acid on a soil clod in a dish and observe the reaction:
- Carbo 3: Strong effervescent reaction (bubbles are large): the soil is calcareous. The active limestone content exceeds 5% and blocking risks are high. No liming planned.
- Carbo 2: An effervescent reaction occurs: the soil is calcareous. The active limestone content is 2 to 5% and there are blocking possibilities. No liming planned.
- Carbo 1: The reaction is very weak (you hear effervescence but see nothing). The active limestone content is below 2% and there is no risk of element blocking.
- Carbo 0: No reaction. No active limestone, the soil is neutral or acidic. Liming is probably needed. The bicarbonate test will clarify this.
3 key points to remember about the calcium cycle
- Calcium is not limestone, but limestone is a calcium reserve.
- Calcium concentration in effluents: Poultry droppings > poultry manure > Compost bovine manure > Bovine manure > Slurry.
- Main effects of calcium: Aeration, soil and plant cell structuring, root growth, and nitrate transformation.



