Liming

Liming is a technique of treatment with lime.
Definition
Soil liming is an agricultural technique that consists of adding calcium or calcium-magnesium amendments to a soil to correct a too acidic pH because excessive soil acidity prevents it from releasing its nutrients to feed plants.
Effects of liming
- It improves soil structure by limiting the risk of forming a crust due to surface sealing by improving the soil's structural stability and its physical properties, notably its "affinity for water." Calcium thus plays an essential role in unstable soils (loamy soils poor in organic matter and clay) not by binding organic matter with clays but by regulating the mobility of metals (cheluviation), including iron involved in organo-mineral bonds. In soils where such bonds have developed, liming improves structural stability; in others, it limits the chemical reactivity of aluminum, iron, and manganese and improves soil microbial activity, which allows better mobilization of nitrogen. However, liming should not be excessive as it can block the mentioned metals and other trace elements as in calcareous soils.
- It compensates for acidification caused by biological activity by increasing a too low pH. Microbial activity necessarily produces organic acids that can inhibit microbial activity and reduce soil fertility if the acids produced are not neutralized. The goal of liming is to neutralize these acids to maintain or intensify microbial activity. This neutralization is not done once and for all but must be renewed every two to three years: shifting from a corrective logic to a maintenance logic.
- It promotes the assimilation of nutrients by plants, particularly trace elements, by compensating for the loss of calcium due to uptake by crops, leaching by percolation water (gravity water), and the effect of fertilizers.
There are 2 types of liming
Corrective liming
To correct an overly acidic soil.
Corrective liming is performed when the [[[Soil pH]]|pHwater]] is below 5.5 and the interval between application and the next crop establishment is short; fast-acting products such as quicklime and pulverized limestones are preferred.
Whatever the crop, pHwater must be above 5.8 (5.5 in sandy soils) to avoid excess acidity detrimental to the crop. Only alfalfa and vegetables have higher thresholds set at 6 and 6.5.
Maintenance liming
To compensate for sources of acidification. Maintenance liming is recommended every 3 to 5 years. In this perspective, slow-acting amendments suffice. To maintain pHwater above the critical threshold of 5.5, the inputs of basic amendments must neutralize the acidity produced. References recommend average annual inputs of 200 to 300 kg CaO/ha or Neutralizing Value units. When plots regularly receive reasoned inputs of organic products, 100 to 200 kg CaO/ha/year are generally sufficient. But only regular monitoring of pHwater allows precise management of maintenance liming. As long as pHwater does not fall below 6, amendment application can wait or be done at a lower dose than planned.
Which type of liming to perform?
The value of pHwater and the crops in the rotation will determine the type of input to provide.
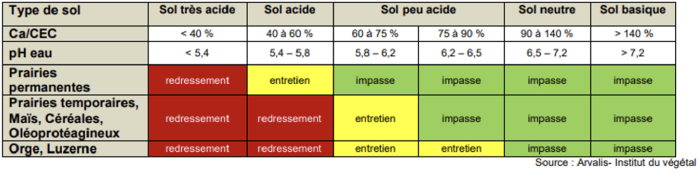
When to lime a soil?
For corrective liming
The calculation of the dose to apply is based on the soil's pHwater but also on its buffering capacity, the CEC.
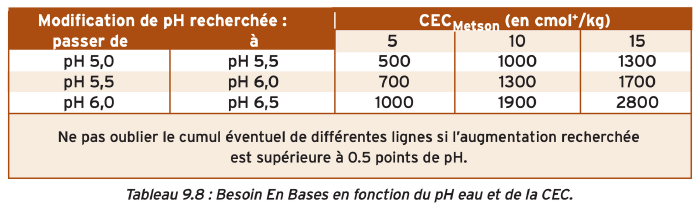
For example, if we have a soil at pH 5.5 that we want to raise to pH 6.5, and it has a CEC of 10, to do this, we add the value to go from 5.5 to 6 (1300) to that from 6 to 6.5 (1900), so a total of 3200 neutralizing value (NV)
For maintenance liming
Regarding maintenance liming, the dose is calculated based on soil type and its theoretical annual loss in neutralizing value due to rain and crop exports.

Liming products
A wide range of basic amendments is available on the market. They present great diversity in physical characteristics (particle size, hardness…) and chemical characteristics (nature of the base and cations, solubility…) as well as very varied prices per unit of Neutralizing Value (NV).
Distinguished are calcined products based on calcium oxides and magnesium oxides (quicklime and magnesian quicklime) and raw products based on calcium and magnesium carbonates. The latter can be more or less fine: pulverized, ground, or crushed and differ by their speed of action depending on their fineness and hardness/softness.
The standard distinguishes 6 classes of basic amendments:
| Class 1
Raw products |
Natural calcium carbonates (CaCO<sub>3</sub>) | Ex: chalk, maerl, marl, limestone amendments |
|---|---|---|
| Class 2
Raw products |
Natural calcium and magnesium carbonates
(CaCO<sub>3</sub> + MgCO<sub>3</sub>) |
Ex: calcium-magnesium amendment, dolomite, magnesium carbonate |
| Class 3
Calcined products |
Lime (calcium oxide CaO and/or
magnesium oxide MgO) |
Ex: quicklime or hydrated lime, calcium and/or magnesian, magnesium oxide |
| Class 4
Mixture raw + calcined |
Mixed basic mineral amendments
(mixture class 3 + 1 or 3 + 2) |
(minimum 15% calcined products) Mixed calcium or magnesium amendments |
| Class 5 | Other basic amendments | Sugar factory scums exclusively |
| Class 6 | Steel industry basic amendments | |
In the table below:
- Neutralizing Value (NV) expresses the potential capacity of a basic amendment to neutralize soil acidity. It depends on the contents of CaO and MgO. Basic amendments, whether calcined or raw, fine or coarse, have the same capacity to neutralize soil acidity, regardless of soil pH; what varies between products is the neutralization delay according to their speed of action. NV is the basis for calculating the dose to apply per hectare and for comparing product prices. If an amendment has a neutralizing value of 92, it has 920 effective units per ton. If this amendment costs €135/ton, then the cost per unit is 135/920 or €0.15 per NV.
- Speed of action refers to the dissolution rate of the product and depends on the product's grinding fineness and the softness/hardness of the carbonate. It is estimated by measuring carbonate solubility. At equal softness/hardness, the finer the product, the more contact it has with the soil and thus acts faster. For a pulverized or ground carbonate, carbonate solubility expresses the speed of action. For a crushed rock, we speak of rock softness/hardness. The rock is soft above 25% carbonate solubility. Speed of action is a criterion for choosing products depending on the type of liming planned (maintenance or corrective).
| Products | NV (neutralizing value) | Speed of action | Price delivered to root in €
per unit of neutralizing value (NV) |
|---|---|---|---|
| Quicklime | 92 | fast | 0.17 - 0.24 |
| Granulated quicklime | 92 | fast | + €0.01 |
| Magnesian lime | 90 | fast | 0.20 - 0.25 |
| Carbonate 54 (pulverized) | 54 | medium to fast | 0.12 - 0.14 |
| Bulk wet carbonates (ground) | 40-50 | medium | 0.08 - 0.1 |
| Calcareous sands, Trez | 30-40 | slow | 0.03 – 0.06 |
Fineness of the product for raw products
At equal hardness, the finer a product is, the faster it acts:
- Pulverized products: 80% of the product passes through a 315 µ sieve
- Ground products: 80% of the product passes through a 4 mm sieve
- Crushed or raw products: more than 20% > 4 mm
Speed of action
It is expressed by:
- Carbonate solubility (CS) for fine products where more than 80% of the product passes through a 4 mm sieve:
- CS > 50: fast action
- 20 < CS < 50: moderately fast action
- CS < 20: slow action
- Rock hardness for coarse products where less than 20% of the product passes through a 4 mm sieve:
- Hardness > 25: soft rock
- Hardness < 25: hard rock
(always > 10)
Sugar factory scums are often used to lime agricultural fields because they also provide phosphorus and trace elements.
In the absence of commercial products, wood ash can be used for light liming.
The special case of organic products
The nitrogen and organic sulfur they contain contribute to soil acidification, but this is more than compensated by organic anions whose effects are similar to those of bases (oxides, carbonates…) contained in mineral basic amendments.
Regular monitoring of pHwater remains the only method usable to take into account the effects of livestock effluents.
Livestock effluents mitigate acidification
Experimental references (Arvalis, IRSTEA, foreign references…) show that farm fertilizers tested almost always help mitigate soil acidification. Thus, trials conducted over 10 years by Arvalis highlighted an improvement in pH with annual inputs of manure, slurry, or composts from livestock, compared to annual mineral nitrogen fertilization only.
In the Jaillière trial (44), six types of effluents applied annually for 10 years on a grassland of perennial ryegrass, based on 150 kg N/ha, were compared to mineral fertilization based on ammonium nitrate (average annual dose of 150 kg N/ha) without liming. After 10 years, the pHwater of the 0-10 cm horizon is significantly higher with organic inputs.
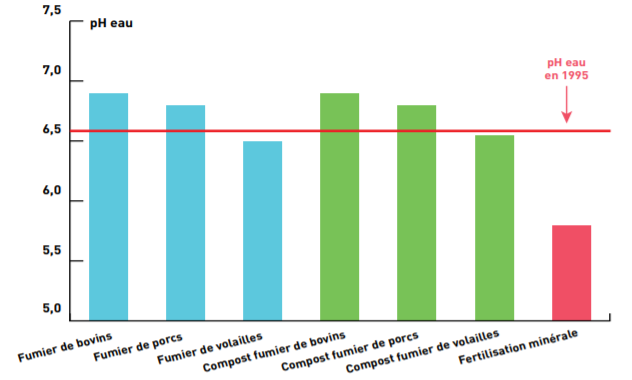
Organic effluent inputs contributed to maintaining pH at 6.6 while the soil acidified with strictly mineral inputs.
Further reading
- Liming dossiers from Semences de France
- Liming - Bourgogne Chamber of Agriculture
- True: pH, a relevant indicator for soil acidity (Arvalis)
Sources
- Wikipedia page on liming
- Maintenance liming: slow-acting products suffice Arvalis-infos
- Liming practice - Chamber of Agriculture Pays de la Loire
- Liming - Chamber of Agriculture Ariège
- Liming: Back to basics - Chamber of Agriculture Brittany