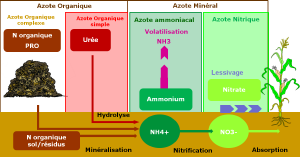
Different forms of nitrogen
Different fertilizers, organic and mineral, contain nitrogen in different forms and proportions. In the soil, nitrate and ammonium are the two main forms of inorganic nitrogen available to the plant. Non-nitrogen-fixing plants absorb and assimilate these two forms of anionic nitrogen (NO3-) and cationic nitrogen (NH4+).
The nitrogen cycle operates between the 3 compartments: soil, air, and water. However, if some transfers are too large, they can have consequences on ecosystem balance. The most impactful fluxes for an ecosystem are: nitrate leaching, with a risk to water quality, and ammonia volatilization, with a risk to air quality.
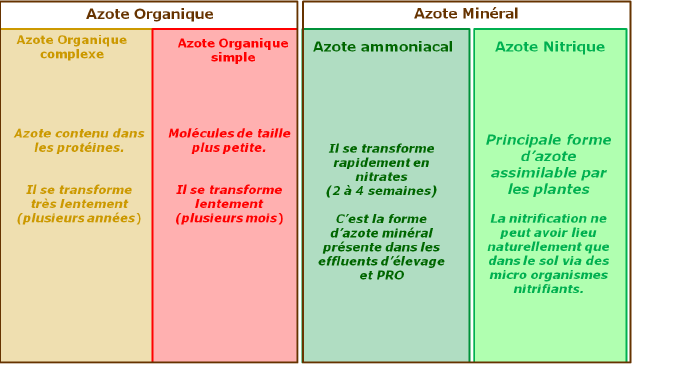
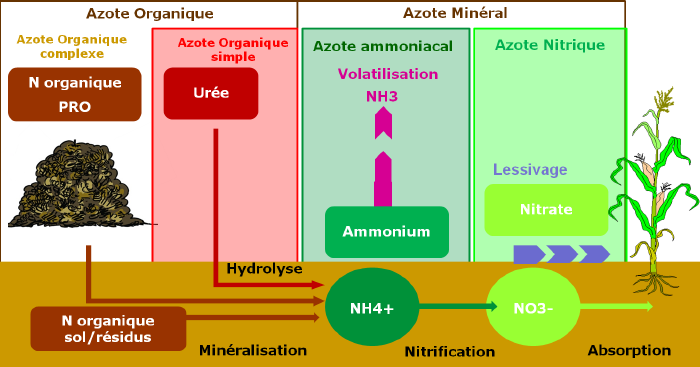
Inorganic nitrogen
Nitrates (NO3-)
This is the main form of mineral nitrogen used by higher plants. Nitrate absorption is an active process that has an energy cost: the plant expends energy to absorb nitrate. Nitrate accumulates in vacuoles up to a limit resulting from a balance with other mineral ions and organic acids. Then, nitrate is reduced to ammonium, in the leaves or roots.
The reduction of nitrates to ammonium occurs in 2 steps:
- Reduction of nitrates to nitrites, by nitrate reductase;
- Then reduction of nitrites to ammonium, by nitrite reductase.
- The overall reaction can be symbolized as: NO3- —> 2e- —> NO2- —> 6e —> NH4+
- During this process, the plant needs cofactors for the enzymatic reaction and thus other mineral elements besides nitrogen:
- Nitrate reductase: requires compounds containing Mo and Co;
- Nitrite reductase: requires compounds containing Fe and S.
This reaction has 3 consequences:
- Increase of soil solution pH, because it consumes H+ ions. Each nitrate ion reduced to ammonia produces an OH− ion. To maintain balanced pH, the plant must either excrete it into the surrounding medium or neutralize it with organic acids. As a result, the medium around plant roots becomes alkaline when they absorb nitrates.
- Turgor = cell swelling. The plant brings water into leaf cells, which leads to thinner walls and increases the risk of attacks by pests (fungal diseases for example).
- Maintenance of ionic balance. Each NO3− absorbed by the root must be accompanied either by absorption of a cation or excretion of an anion. Plants can absorb cations such as K+, Na+, Ca2+, and Mg2+ to exactly match each nitrate absorbed and store them as salts of organic acids like malate and oxalate. If these cations are lacking to maintain balance, a deficiency may appear in this reduction process. Nitrate accumulation in the plant can influence plant sensitivity to pathogens.
Ammonium (NH4+)
Ammonium is mainly assimilated by the roots. It is incorporated into amino acids by glutamine synthetase and glutamate synthase (enzymes derived from amino acids).
- NH4+ comes from the degradation of organic nitrogen.
- NH4+ is energy-rich and is rapidly used by nitrifying bacteria.
- These transformations can be inhibited if bacterial metabolism is inhibited (heat, too acidic pH, lack of oxygen).
NH4+ absorption can have two effects:
- Acidification of the medium: the pH gradient around the root affects the microflora. Microflora is scarce in acidic environments, so there will be little transformation of mineral nitrogen into organic nitrogen.
- Ammoniacal syndrome: when ammoniacal nitrogen is abundant in the soil, the plant preferentially feeds on ammonium. Since ammonium is a cation, its presence causes competition with other cations (calcium, potassium, magnesium). A balance is not established and the nutrition of crops is impacted. For example, a maize seedling preferentially absorbs ammoniacal nitrogen rather than nitrate nitrogen, especially when soil content in Ca2+, K+, Mg2+ is low. Fertilization based on ammonia, urea, or organic amendment in low temperature contexts does not favor transformation of ammoniacal nitrogen into nitrate nitrogen (nitrification). Low radiation during this 3 to 6 leaf stage does not allow sufficient photosynthetic activity and metabolization of absorbed ammoniacal nitrogen.
- Ammonium absorption allows absorption of free sugars, which activate photosynthesis.
Mixed nutrition
Dual absorption (NO3- / NH4+) allows pH balance. Mixed nutrition solves the problem of rhizosphere acidification.
Urea
Urea must first be transformed into ammonium by an enzyme, urease. The transformation rate depends on soil temperature. At 10°C, the process takes a few days. This duration increases if temperature decreases. Thus, if urea is spread on cold soil, it remains stable and unavailable to plants. When temperatures rise, plant growth starts and urea transformation into ammonium increases.
- Transformed into ammonium, part is fixed to the clay-humus complex and another part is transformed into nitrate by microorganisms. Urea mineralization in soil starts in spring, as soon as soil temperature increases.
- Wheat and maize can absorb urea in its molecular form and use urea nitrogen for growth. In cereals, urea is the least efficient nitrogen source in terms of yield compared to ammonium and nitrate. Plants fed with urea as the sole nitrogen source also show characteristic symptoms of nitrogen limitation. This nitrogen deficiency is not due to an inability of plants to assimilate nitrogen from urea but rather to a lower efficiency of urea absorption by roots.
Organic nitrogen
For a long time, it was assumed that inorganic nitrogen (in the form of NH4+ and NO3−) was the only nitrogen source for plants. However, for 20 years, research has shown that amino acids are now treated as another nitrogen source for most plants (study by GIOSEFFI et al., published in 2011 titled "Interactions between amino acid and inorganic nitrogen absorption for wheat" concluding that organic nitrogen can be an important nitrogen source for wheat and that there is interaction between inorganic and organic nitrogen absorption).
Efficiency and assimilation speed
The most easily assimilable form by plants, without transformation, is the nitrate ion NO3-. Other forms require chemical transformation by microorganisms to reach the form of nitrate ion: this is the case for ammonium ion NH4+ (mineral nitrogen) and organic nitrogen (urea, etc.).
The speed of this transformation depends on soil temperature. This nitrogen thus has a delay effect and the supply must be anticipated. In well-aerated soils, nitrification is rapid: resulting in low ammonium concentration, nitrate being the main nitrogen source available to plants. In waterlogged or acidic soils, ammonium accumulates.
Here is a graph from a 2013 Arvalis trial measuring the efficiency of different nitrogen fertilizer forms on soft wheat: ammonium nitrate, urea and Nexen (urea + nitrification inhibitor).
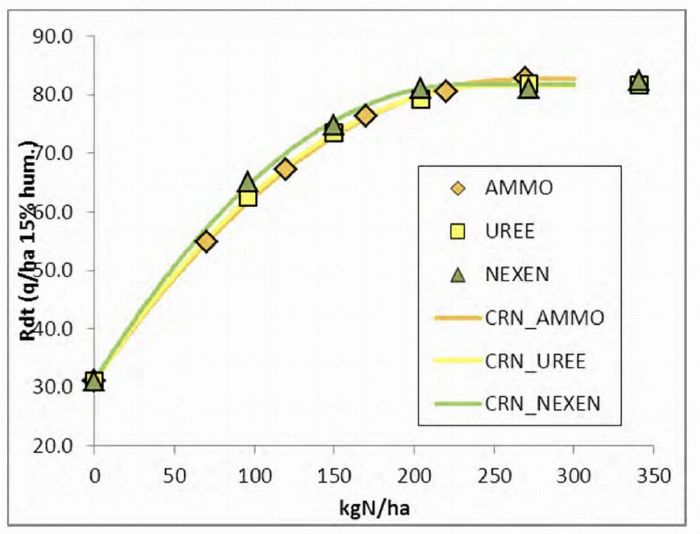
- It is noted that ammonium nitrate and Nexen are slightly above urea in terms of efficiency.
- However, urea is almost at the same level when the fertilizer is well positioned.
Distribution of nitrogen in different effluents
In organic fertilizers, nitrogen is organized into three forms:
- A mineral fraction which has a short-term effect (the first year after spreading).
- A rapidly mineralizable organic fraction which partly has a direct effect and partly a short-term residual effect.
- A more stable organic fraction with long-term effects.
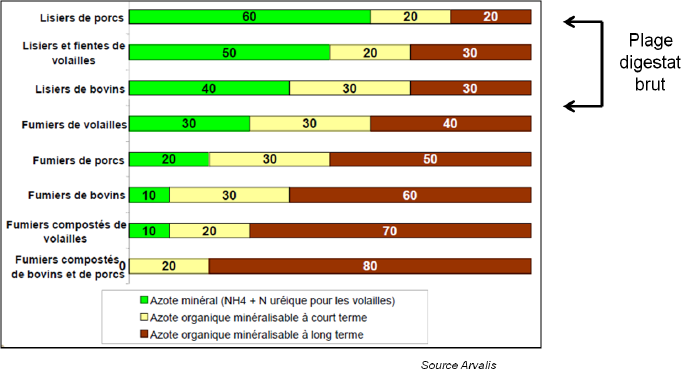
The more composted a manure is, the more it contains a stable organic fraction with long-term effect. Slurries have a greater direct effect on plants due to a larger mineral fraction.
Equivalence coefficient
Fertilizing effects on the receiving crop are estimated by the percentage of the considered element (N, P2O5, K2O, MgO, SO3) apparently used by the plant (CAU) and by the equivalence to a reference mineral fertilizer for this element (Keq). The reference mineral fertilizer is ammonium nitrate for nitrogen, Super 45 for phosphorus, potassium chloride for potassium, and magnesium sulfate for magnesium.
The equivalence coefficient corresponds to the fertilizer equivalence of one kilogram of element (N, P, K,…) supplied by Organic Residual Products (ORP). This coefficient is obtained using a response curve of element uptake as a function of increasing doses of this element applied as soluble mineral fertilizer and the amount of element absorbed by the crop fertilized with an ORP dose.
Keq is higher when the ORP contains mineral nitrogen and rapidly mineralizable organic nitrogen. It also depends on the receiving crop, the timing of application, and whether it is incorporated or not. In practice, the total dose of fertilizing element supplied must be multiplied by the mineral fertilizer equivalence coefficient to obtain the actual supply of fertilizing elements to the crop.
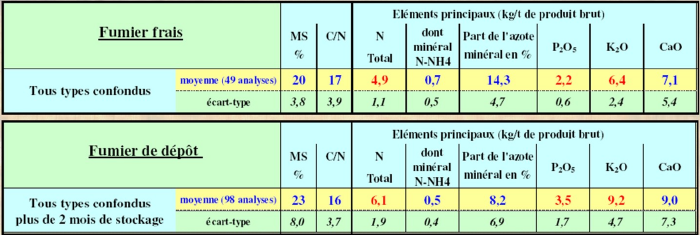
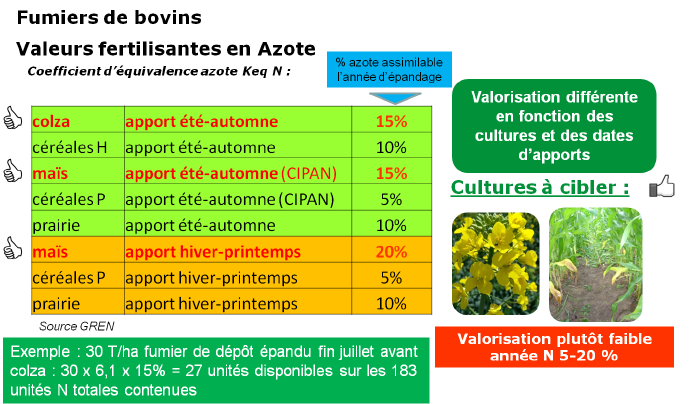
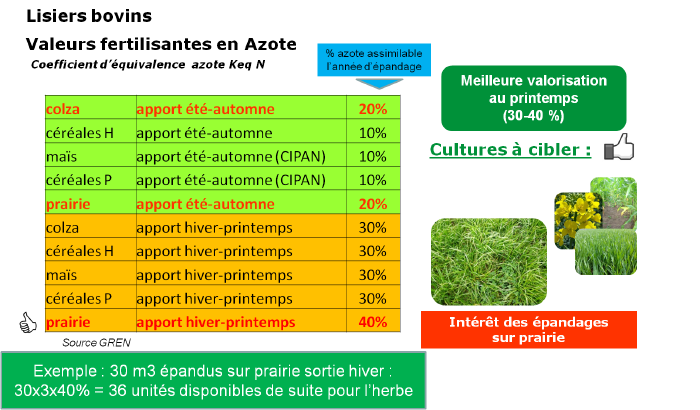
Cattle manures
Cattle manures have very different characteristics depending on the farming system; it is necessary to have them analyzed. Analysis results depend on animal types, housing, and manure storage. Manures also contribute to base fertilization of fields as they are rich in potash and phosphorus.
The more composted the manure, the higher the total nitrogen proportion, but it is stable nitrogen that contributes to humus formation in the soil.
Manures are well valorized by rotation crops such as rapeseed or maize. A large part of the nitrogen present is however not immediately assimilable after spreading but will gradually amend the soil.
Caution, in grasslands manure spreading can cause a decrease in palatability if the manure is poorly degraded.
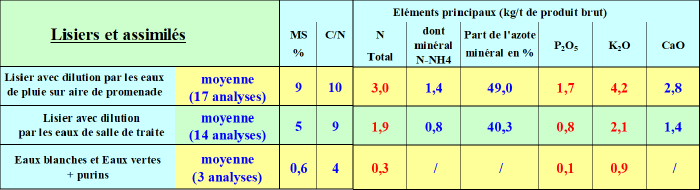
Cattle slurries

Again, analysis will allow knowing its effluent. Slurry pits may be covered or not and dilution effects will impact the nitrogen content. The mineral nitrogen fraction, thus directly assimilable by the plant, is close to 50%, the fertilizing effect is direct.
Slurries are better valorized in spring, when leaching risk is lower.
Whether in autumn or spring, grasslands best valorize slurries. An application can also be made before rapeseed to create a starter effect on the crop.
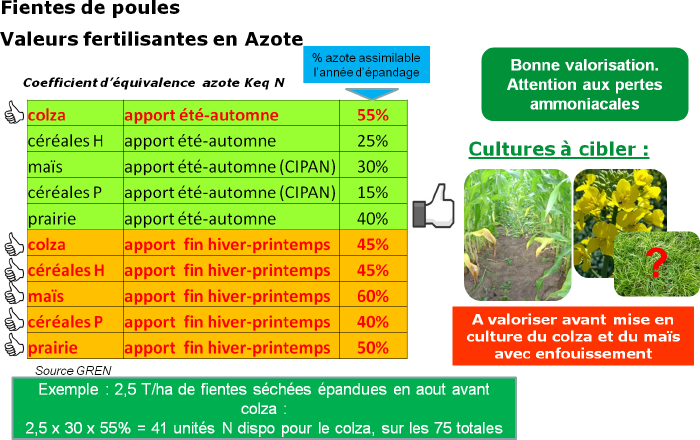
Poultry droppings
Poultry droppings are very rich in nitrogen, they also contain phosphorus, potassium, and calcium. Poultry droppings are very rapidly mineralized in the soil: nitrogen is almost immediately available to plants. The drier the poultry droppings, the more concentrated the nitrogen.
High ammoniacal nitrogen content in liquid or semi-solid livestock effluents causes the risk of losing up to 70% of nitrogen after spreading by ammonia volatilization. These losses are economically and especially environmentally harmful: ammonia reacts with acidic compounds to form very fine particles of nitrates or ammonium sulfate responsible for soil acidification and eutrophication of environments. Rapid incorporation after spreading is therefore recommended to reduce nitrogen losses: the gain is estimated at 60% of total losses for immediate plowing after spreading.
| Poultry droppings | Total N | P205 | K2O | % DM |
|---|---|---|---|---|
| Wet poultry droppings | 15 | 14 | 12 | 25 |
| Pre-dried poultry droppings | 22 | 20 | 12 | 40 |
| Dried poultry droppings | 30 | 40 | 28 | 80 |
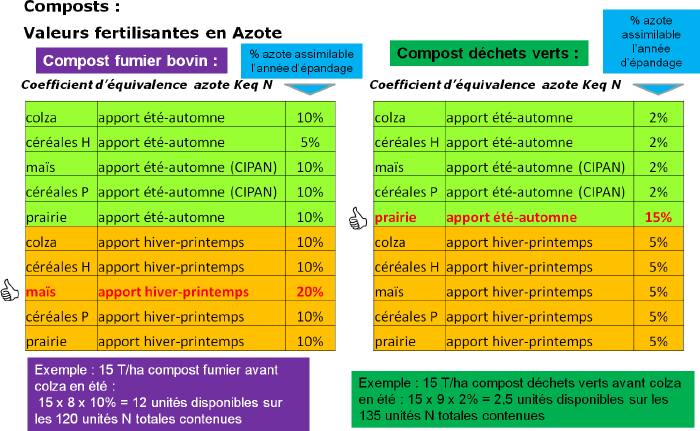
Composts
- The young manure compost, obtained by short composting (generally between 1 and 3 months), is rich in nitrogen and polysaccharides (provided by straw). It is a "starter" product behaving like a fertilizer and strongly stimulates soil microbial activity. Thanks to its action on microbial activity, it also improves soil structure and reduces element blocking phenomena in calcareous soils. Preferably applied at the end of winter.
- The mature manure compost, obtained by long composting (several months), is a slow-degrading product behaving like an amendment and provides much fewer fertilizing elements. It has less effect on soil microbial activity than young compost but increases the stable humus content more.. It thus acts on soil structure and improves water retention capacity.
- Green waste compost is characterized by a strong amendment effect and low fertilizing power. It has a slow evolution dynamic and great stability, and tends to increase the soil organic carbon stock.
| COMPOSTS | Total nitrogen |
|---|---|
| Cattle manure compost | 8 kg/t |
| Poultry droppings compost | 15 kg/t |
| Green waste compost | 9 kg/t |
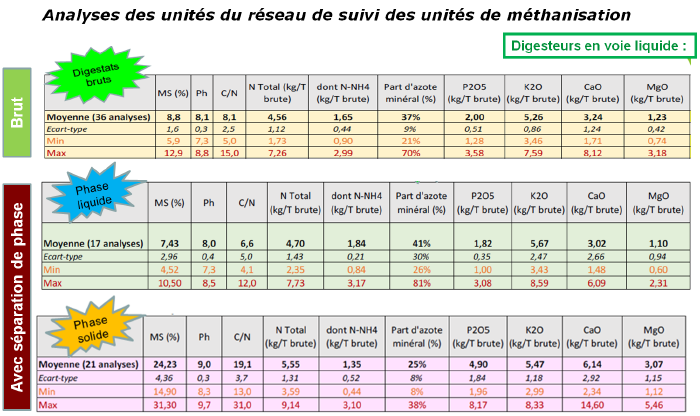
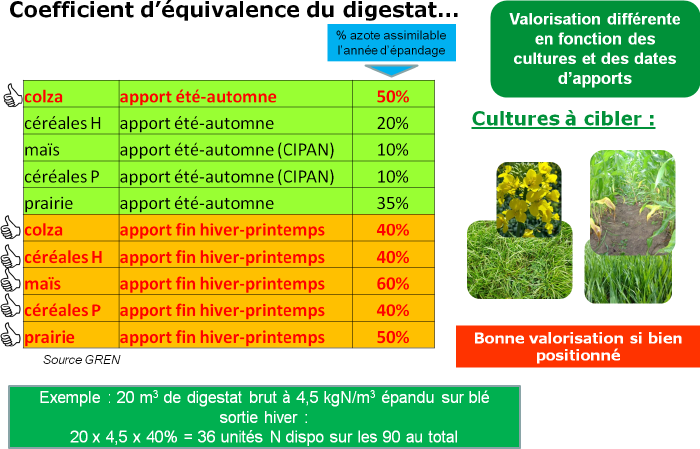
Methanization digestates
As with manures or slurries, methanization digestates have 3 different nitrogen fractions: mineral nitrogen, organic nitrogen mineralizable within the year and organic nitrogen mineralizable in following years, and as with other effluents, each digestate is unique and requires analysis.
During phase separation, the mineral fraction concentrates in the liquid phase. Digestate is very well valorized at winter end as a replacement for the first application.
For autumn applications, preference is given to applications on rapeseed. It is also possible to apply on CIPAN (Catch Intermediate Crop Nitrate Trap) which valorizes nitrogen well, but only if it emerges properly. The CIPAN will return nitrogen to the spring crop which will valorize it in turn. It is essential to incorporate the digestate if applied before sowing, to limit ammoniacal nitrogen losses by volatilization. However, be careful with application doses during this period as needs are reduced.
Sources
- Different forms of nitrogen - Chamber of Agriculture of Meuse: https://meuse.chambre-agriculture.fr/fileadmin/user_upload/Grand-Est/037_Inst-Meuse/Eau/NT7-RDM-valeur_de_la_MO.pdf
- Different forms of nitrogen, AgroLeague
- Techniques to optimize nitrogen inputs, AgroLeague
La version initiale de cet article a été rédigée par Camille Crespe, Julien Basuyaux, Ludovic Purson, Lorraine Briard et Jean-Luc Lefevre.