 Bioelectronics, Oxydo-reduction, Principles and effects of RedOx potential, Impacts of agricultural practices on redox
Bioelectronics, Oxydo-reduction, Principles and effects of RedOx potential, Impacts of agricultural practices on redox
Redox potential is a good indicator of the health of a soil or plant. Redox mechanisms regulate everything in the plant, the soil and living organisms. They regulate physiology (nutrition, immunity, cellular processes, greenhouse gas emissions, etc.), phenology and interactions with pests.
A plant and its soil need a good redox balance to develop and resist attack. This balance is provided by the physical structure of the soil and by the diversity of soil and root life found there.
This article explains how redox works and how a plant reacts to variations in it. The functioning of an electric battery is a good illustration of the phenomenon.
General information
It is very important to note that redox potential and pH are dependent on each other, and that a redox potential value is only meaningful if it is accompanied by the pH value of the medium in which it was measured.
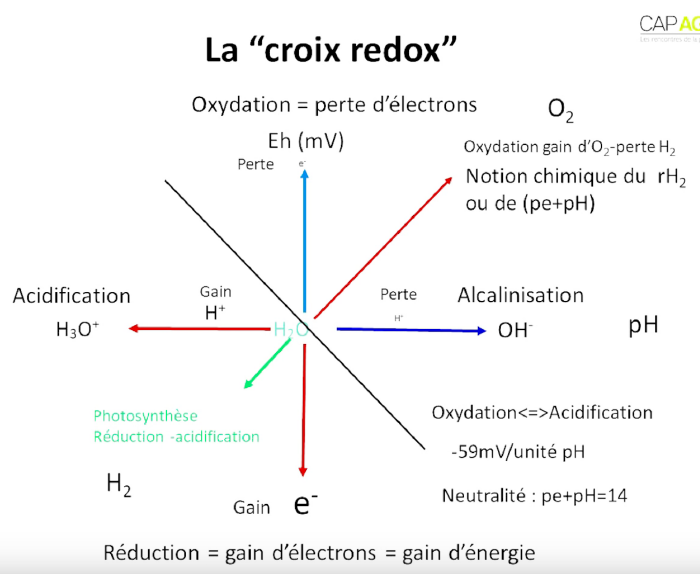
The redox potential can be found under 2 names :
- Eh when expressed in Volts or mV.
- pE when it is expressed on a scale (similar to the pH scale) that expresses electron activity.
The neutral redox potential can be considered to be at 400mV :
- Eh>400mV : oxidised state.
- Eh<400mV : reduced state.
Most reactions in a living cell are acid/base (pH) and/or oxidation/reduction (Eh), and the two are interdependent. This is why we look at the diagonal axes of the redox cross (Eh+pH) instead of the pH and redox potential (Eh) axes alone.
Chemists and biologists do not have the same definition of redox, but both are correct, depending on the point of view.
- According to the biologist : oxidation = loss of hydrogen = gain of oxygen.
- According to the chemist : oxidation = loss of electron.
In acid/base reactions, protons are exchanged, whereas in redox reactions, electrons are exchanged. The protons + electrons that make up the energy are hydrogen (which is made up of a proton and an electron). By storing energy in the form of hydrogen, the plant can be compared to a hydrogen fuel cell.
During photosynthesis, water is hydrolysed, which means that hydrogen (energy) is recovered. This hydrogen is then attached to carbon. If we keep the image of the hydrogen fuel cell, we can compare the carbon to the compartment of a battery whose energy source is hydrogen.
If we look at the diagram of the redox cross : in the bottom left-hand corner there is a lot of energy and in the top right-hand corner there is none. However, you mustn't go too far to the bottom left because you won't have enough energy left to use. There has to be a balance between hydrogen (fuel) and oxygen (oxidant) for the system to work.
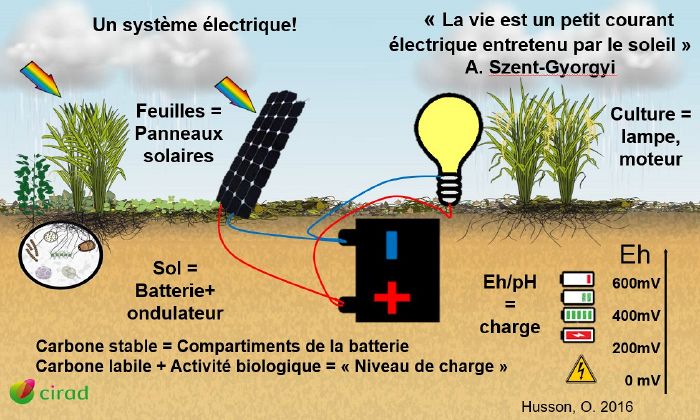
Oxidation-reduction and energy transfer in a crop
As we have just seen, photosynthesis enables plants to fix solar energy by reduction (=gain of hydrogen thanks to the hydrolysis of water). This energy is stored in the form of matter in the living and dead biomass of the soil. The more healthy vegetation there is, the more photosynthesis there will be, and therefore the more energy stored.
Through root exudation, plants feed the soil microorganisms. This healthy microfauna creates good soil conditions :
- good structure
- digestion of organic matter
- release of nutrients that can be used by plants.
The result is a healthy soil, conducive to the production of significant plant biomass.
These root exudates release energy for the plant, which also has the effect oflocally adapting the pH andEh to its needs. In some cases, this expenditure represents up to 80% of the plant's energy requirements.[1] of the energy produced by photosynthesis.
All plant metabolic reactions are controlled by pH and redox. They are therefore obliged to maintain specific pH/Eh levels in each cellular compartment, and to do this they also need the soil to be within a certain pH/Eh range.
Cultivated soils have pH values of 4 to 9 and average redox potentials of between -300mV (highly reduced) and +900mV (highly oxidised).[2]but most of them are in fact oxidised when it's dry and asphyxiated when it rains.
The right situation for plants is at the "bottom left" of the redox cross diagram (but not too high either, otherwise it corresponds to an oxygen-free environment, such as a rice paddy).
Feeding and assimilation of soil nutrients
The pH/Eh conditions determine the form in which nutrients are available and therefore the ease with which plants can assimilate them. Plants are then able to modify the pH/Eh conditions locally around their roots in order to feed themselves.
Pourbaix diagrams show the species present as a function of redox potential and pH.
Pourbaix diagram for nitrogen
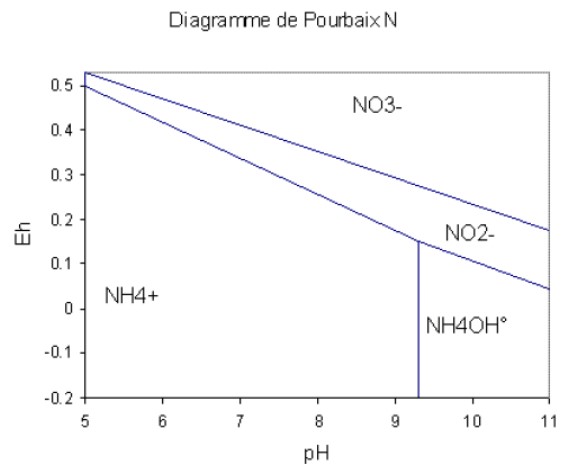
Nitrogen is best assimilated in an organic form, but it is generally a mineral form that is present in the soil. The oxidised form of nitrogen is nitrate (NO3-), the reduced form is ammonium (NH4+).
Ammonium and nitrates tend to imbalance the plant's electrical charge and therefore its pH. The plant balances the pH by itself through physiological mechanisms (proton pumps), but this costs energy. Ideally, it absorbs ammonium and nitrate at the same time so that they compensate for each other, but in unbalanced soils, it can only access one of the two forms.
Pourbaix diagram for iron
The Pourbaix diagram for iron is also interesting to look at, as iron is an essential element that blocks rapidly as soon as the soil is oxidised.

Soil structure is fundamental
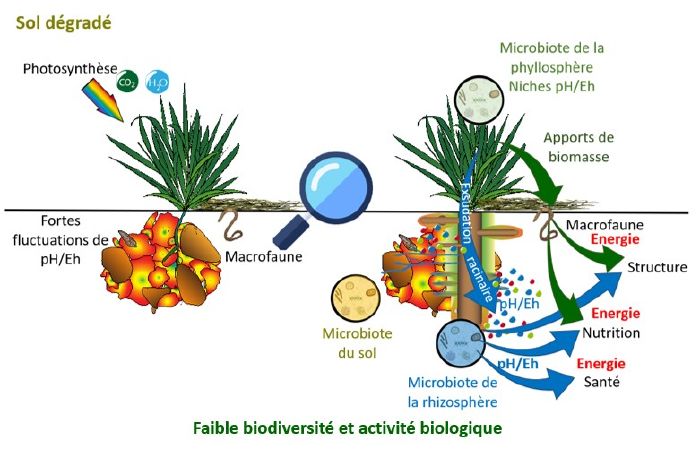
- Well-structured soil allows air and water to circulate, resulting in a wide range of pH/Eh niches in space that change little over time. This is very favourable to biological activity (presence of several types of micro-organisms) and to the bio-geo-chemical cycles of the various elements, because a plant will always be able to "put down a root" in an aggregate that provides it with what it needs (iron, manganese, nitrates, etc.), natural antibiotics, amino acids and various forms of protection.
- On compacted soil (where water and air circulate poorly), when it dries out, the form of nitrogen present will be nitrate (NO3-) and as soon as it rains, the soil will asphyxiate and the form present will be ammonium (NH4+). This type of soil will therefore be considered oxidised in the dry season, but in the autumn it will be too reduced.
Physics (soil structure) dominates chemistry (form of the element present in the soil) and biology (micro-organisms present in the soil and therefore plant development).
Each plant can only develop properly under certain pH and redox conditions. The optimum for most plants is :
- pH = 6.5 to 7.
- Eh = 400 to 450 mV.
Reducing conditions are particularly limiting, although some plants, such as rice, can grow in very reducing conditions. TheEh of submerged rice field soil can reach -200mV. Rice is able to resist this by increasing the redox potential locally around its roots, by sending oxygen from its aerial organs.
Soil oxidation factors
- As oxygen (O2) is a very good oxidant, the level of oxidation in the soil will depend heavily on its oxygenation and therefore its porosity and water content. It also depends on theactivity of aerobic organisms, which consume O2.
- The organic matter plays an important role. Fresh organic matter is reduced and is an important source of electrons. It acts as a very good buffer, preventing both hyper-oxidation and hyper-reduction. The redox potential of fresh straw, for example, is around 150mV[2].

Diagram of the molecular structure of a clay - Clay content : the iron in the clay sheets can accept or donate electrons, and the clay helps to form the clay-humus complex which is the basis of good soil structure. It is more difficult on very sandy soils to maintain a good structure.
Health and the plant's ability to defend itself against aggressors
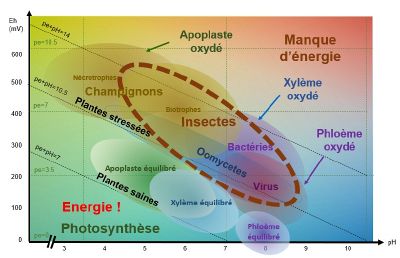
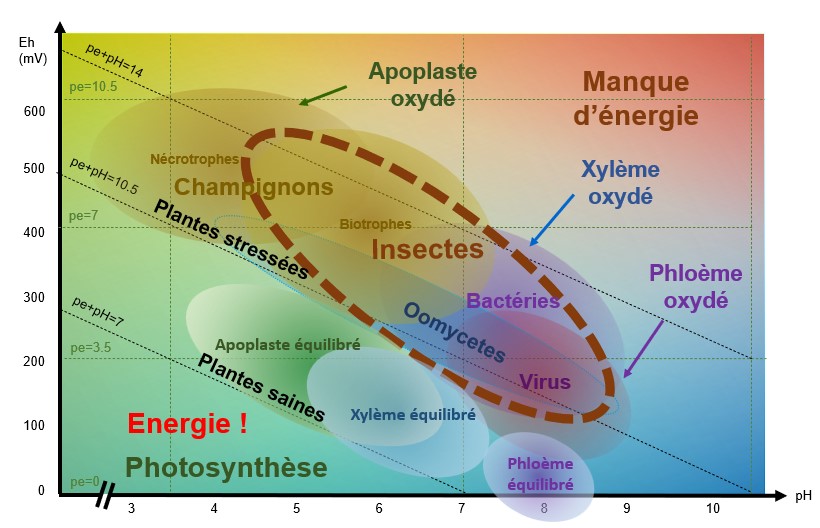
Like plants, insects, fungi and bacteria need an optimum pH/Eh balance to function. Pathogen balances all fall within a zone that corresponds to a state of stress for the plant. When the system is low in energy, i.e. oxidised, conditions are conducive to the development of pathogens. And the plant does not have enough energy available to re-establish "healthier" reducing conditions.
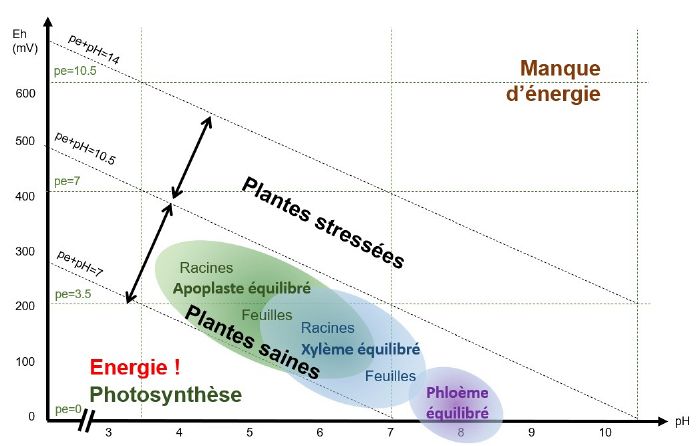
When pE+pH is between 7 and 9-10 (depending on the species), the apoplast (extra-cellular medium), the xylem (which carries the raw sap) and the phloem (which carries the processed sap) are balanced and the plant is able to defend itself.
As plants age, they acidify and oxidise, becoming less susceptible to bacteria and more susceptible to fungi. Periods of stress such as cold, shade and deficiencies reduce photosynthetic activity and causetissue oxidation, which increases the risk of infection by fungi and viruses.
pH/Eh conditions favourable to the development of fungi
When theapoplast is oxidised, i.e. when conditions are acidic and oxidised, necrophagous and biotrophic fungi find themselves in pH/Eh conditions that are favourable to their development.
For example : Botrytis cirenea develops when the pH is between 5-6 and theEh between 500-550 mV.
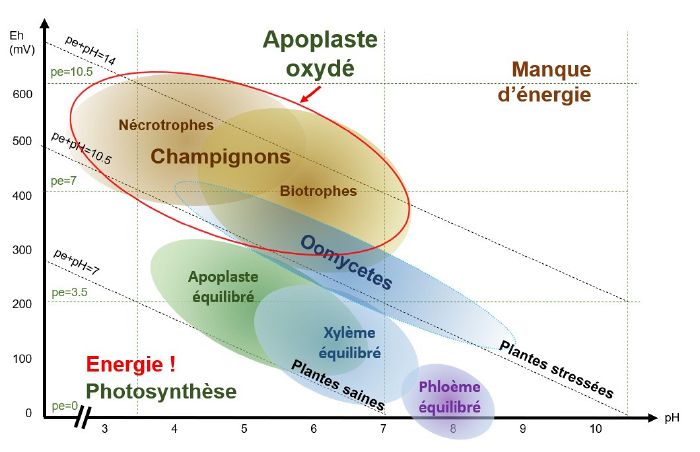
pH/Eh conditions favourable to the development of bacteria and oomycetes
When the xylem is oxidised, i.e. when conditions are slightly acidic to slightly basic (depending on the species and the type of stress) and the pE+pH>10, bacteria and oomycetes find themselves in pH/Eh conditions that are favourable to their development.
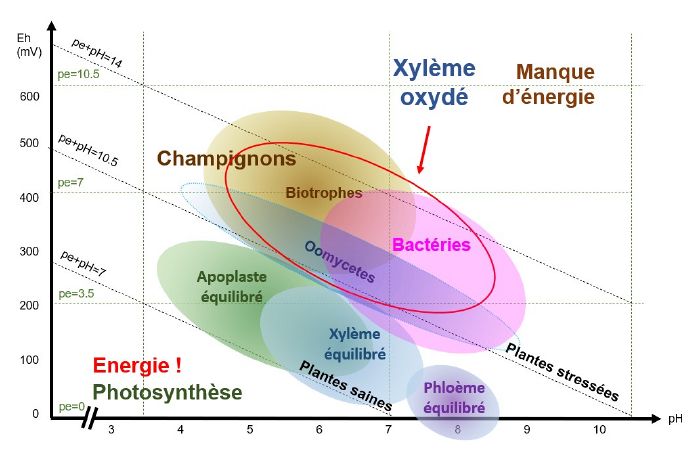
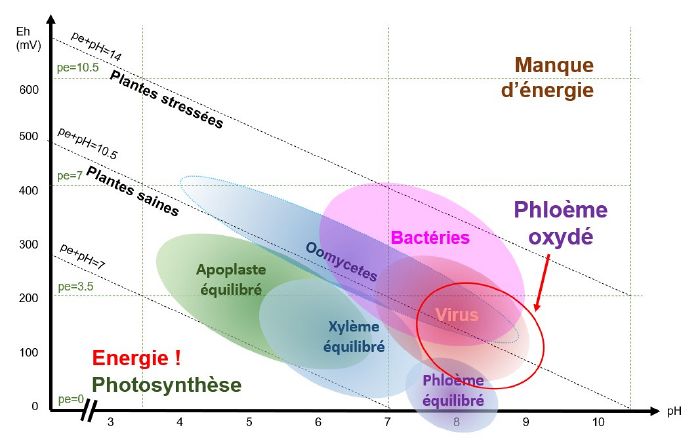
pH/Eh conditions favourable to the development of viruses
When the phloem is oxidised, i.e. when conditions are basic and oxidised and pE+pH>9 - 10 (depending on the species), viruses find themselves in pH/Eh conditions that are favourable to their development.
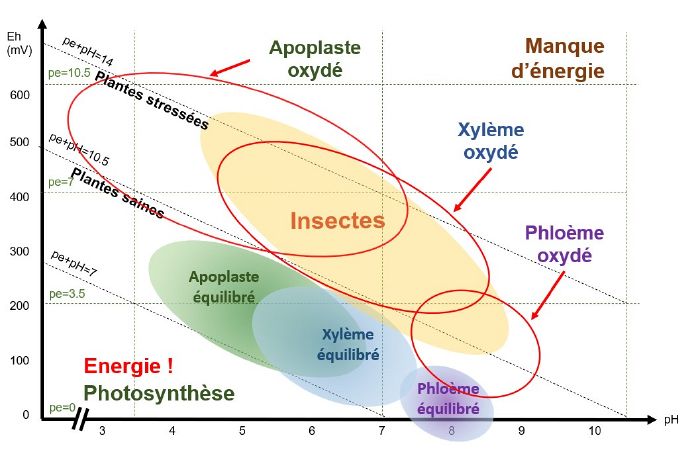
pH/Eh conditions favourable to insect development
Insects will attack a plant that is oxidised pE+pH>9-10 (depending on the species).
Plant defences
Plants can defend themselves against these attacks by modulating their redox potential or pH. However, they often do not have enough energy and electrons to create reducing conditions. In these cases, the solution is to create local hyper-oxidation, like a backfire, by producing hyper-oxidised molecules such as hydrogen peroxide (H2O2) and concentrating them around the fungus. This also kills the plant cells but limits infection. This is also how most fungicides work.
It is therefore possible to prevent crop diseases by promoting plant defence mechanisms, thanks to slightly reduced and acidic soils, which are therefore charged with electrons and energy. If the soil's redox potential is already favourable, plants don 't need to send the molecules and energy produced by photosynthesis toadjust it. Instead, they can keep everything to themselves and thus fight against pathogens that like oxidised environments.
How can we promote the health of plants ?
By encouraging a system full of energy through permanent cover and good soil structure !
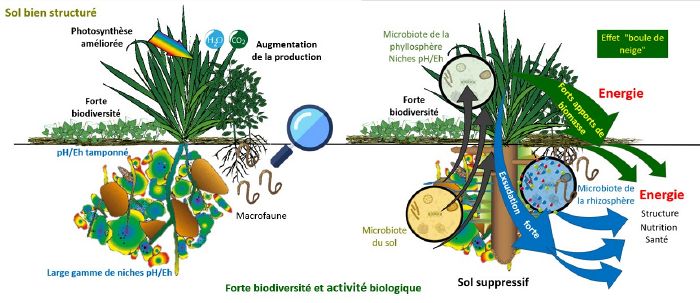
We saw above how healthy soil and healthy plants support each other.
- A healthy soil full of energy is obtained by photosynthesis (just like charging a battery with a lot of solar panels).
- Healthy soil is porous, allowing water and air (and therefore hydrogen and oxygen) to circulate.
- It is full of niches in which the pH/Eh conditions are different, allowing for different micro-organisms and nutrients, so at the level of its root network, the plant has everything it needs to grow. Soil pH/Eh conditions are highly variable in space but not in time.
- Organic matter and clays play an important buffering role.
Favourable and unfavourable practices
Techniques that oxidise the soil
Action of tillage (injection of oxygen, oxidation of OM)
Action of the sun (dry // hot)
Action of rain (contains dissolved oxygen)
Fertilisation with oxygen (NO3-, SO3, SO4, P2O5, K2O, CaO, MgO, ...)
All phytosanitary products (CuSO4, herbicides, fungicides, insecticides)
Techniques that reduce soil
Action of photosynthesis (recovery of electrons through the Calvin cycle)
Action of residues (straw, wood, leaves, roots, humus, soil OM)
Action of effluents (manure, compost, slurry, digestate)
Effect of lack of oxygen (hydromorphy, compaction, soil type)
All oxygen-free fertilisers (NH4+, NH2, NH3, oligo, etc.)
All organic acids (ascorbic, lactic, humic, fulvic, citric ?, acetic ? aspirin ?)
The redox potential of a soil
Measuring the redox potential of a soil is of little use, as it varies greatly in space and time depending on its structure. It's better to measure a plant's redox potential because it's a good indicator of its state of health.
Impact of farming practices on soil redox potential
Most conventional farming practices oxidise the soil :
- Tillage : ploughing will incorporate oxygen into the soil and remove plants that photosynthesise.
- Inputs : fertilisers (nitrates, chlorides, etc.) are oxidising, while herbicides, fungicides and insecticides have a hyper-oxidising effect.
- Spraying : will incorporate oxygen into the inputs, which are themselves already oxidising.
- The absence of ground cover : the sun's rays on bare soil strongly oxidise the soil. Mulching or covering the soil limits oxidation.
What's more, these conventional practices destructure the soil, leading to compacted soils with wide fluctuations in pH/Eh conditions over time, which is highly unfavourable to biodiversity and plant growth.
Undisturbed soil covered with vegetation is regularly supplied with electrons (and therefore reduced matter) by plants. There is therefore a significant redox potential gradient between the surface of the soil and the lower layers. Ploughing destroys this gradient by turning the soil over, which disrupts the functioning of the microfauna and the movement of ions(K+, Na+...) necessary for crops and pH regulation.
A redox potential that is favourable to crops is stable (frequent variations are very energy-consuming for plants) and can be encouraged by a stable structure that allows water to be stored and air to circulate, and therefore by practices such as :
- Not turning over the soil.
- Liveplant cover.
- Restoring biomass[3]].
Measuring a plant's redox potential
Redox meter
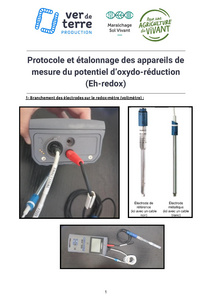
To measure the redox potential in a plant, you need a redox meter, which is in fact a voltmeter. The measurement protocol is explained in this document :
Near infrared spectrometry

The electrochemical measurements described above are time-consuming and delicate. Faster methods based on near infrared spectrometry have recently been developed. This is the case with the Food Scanner developed by Senseen.
This tool is a miniaturised near infrared spectrometer (NIRS) that measures plant stress and enables agro-ecological systems to be managed using redox potential, pH and conductivity
(EC). By simply scanning products directly in the field, it is possible to assess the condition of soils, vines, wheat and oilseed rape (for the moment) and obtain measurements predicted by artificial intelligence. This data can then be linked to decision-making tools.
In a nutshell
- Energy comes from photosynthesis.
- A well-structured soil offers a wide variety of pH/Eh niches for plants, enabling them to feed better, be healthier and produce more.
- Soil and plants are constantly trying to balance each other out- this is the phenomenon ofhomeostasis. Internally : in the plant's life cycle and/or externally : in the face of variations in climate, types of damage caused by farming practices, pests, etc.
- The healthier the soil/plant system, the better it will be able to react. When there are too many imbalances, this opens the door to pathogens and other attacks. Life, whether in the soil or in the plant, will try to compensate for imbalances by readjusting them, an action that consumes a lot of energy, energy that will not be available for anything else.
- The pH/Eh is constantly changing. It is therefore all the more useful to know what is affecting the system in terms of oxidation or reduction, acidification or alkalinisation, so that we can reduce as far as possible what is damaging the system. The healthier the system, the more reactive it is at a lower energy cost.
- You need healthy soil to have healthy plants, but you can't have healthy soil without healthy plants !
Effect ofEh redox potential on pollution
Pollution by nitrates and nitrous oxide
- In an oxidised medium (Eh>500mV at pH 7[2]), nitrates (NO3-) are the predominant form ofnitrogen. It is also a highly mobile molecule , sensitive to leaching. There is therefore a significant risk of pollution of water tables and crop deficiencies.

- In environments that alternate between flooding and drainage (and therefore between reduction and oxidation), as is the case in rice-growing for example, there are also major losses of nitrogen. When the soil is drained, the nitrogen is oxidised to nitrate, then when the soil is flooded, the nitrates are transformed via denitrification into two gases that escape from the soil : nitrous oxide N2 or nitrous oxide N2O, which is a powerful greenhouse gas. The faster the alternation, the greater the losses.[4]. Losses in the form of nitrogen are greatest forEh<0mV[5] and are simply a loss of nutrients for crops. Losses in the form of nitrous oxide, on the other hand, are greatest aroundEh=0mV[5]are also a source of atmospheric pollution.
Training
This article was written with the kind assistance ofOlivier Husson - CIRAD.
See the full training course :
- 1/7 : Introduction au Redox-pH en agriculture
- 2/7 : Redox-pH et fonctionnements des sols
- 3/7 : Redox-pH et fonctionnement des plantes et de la vigne
- 4/7 : Redox-pH et santé des plantes et de la vigne
- 5/7 : Impacts des pratiques agricoles sur le Redox-pH
- 6/7 : Redox-pH, vinification et mesures terrain
- 7/7 : Redox-pH et santé des animaux
To find out more
- https://www.researchgate.net/publication/233729357_Redox_potential_Eh_and_pH_as_drivers_of_soilplantmicroorganism_systems_A_transdisciplinary_overview_pointing_to_integrative_opportunities_for_agronomy Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy - Olivier Husson].
- Soil and plant health in relation to dynamic sustainment of Eh and pH homeostasis: A review - Olivier Husson, Jean-Pierre Sarthou, Lydia Bousset, Alain Ratnadass, Hans-Peter Schmidt, John Kempf, Benoit Husson, Sophie Tingry, Jean-Noël Aubertot, Jean-Philippe Deguine, François-Régis Goebel & Jay Ram Lamichhane
Sources
- Interview with Olivier Husson on 30/07/2022.
- Soil health through plants - Samekh Centre.
- Sarah E.Johnson-BeeboutOlivyn R.AngelesMaria Carmelita R.AlbertoRoland J.Buresh (2009), Simultaneous minimization of nitrous oxide and methane emission from rice paddy soils is improbable due to redox potential changes with depth in a greenhouse experiment without plants, Geoderma Vol 149, Issues 1-2, 15 February 2009, Pages 45-53 https://doi.org/10.1016/j.geoderma.2008.11.012
- Ver de Terre Production (13 May 2019) RedOx, pH, resistivity in Living Soil, https://www.verdeterreprod.fr/redox-ph-du-sol-et-des-plantes/
- Wacker L. (9 May 2022) Soil redox potential influences plant growth, AgroLeague
- Wacker L. (9 May 2022) Redox - episode 2 : the forgotten parameter of fungal diseases, AgroLeague
- ↑ Husson Olivier - pH and ORP in agriculture
- ↑ 2.0 2.1 2.2 Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 362, 389-417 (2013). https://doi.org/10.1007/s11104-012-1429-7 https://link.springer.com/article/10.1007/s11104-012-1429-7#citeas
- ↑ Singla S., Husson O. (September/October 2018) Redox potential - Life is a small electric current powered by the sun, AGRONOMY, ECOLOGY AND INNOVATION. TCS N°99 https://agriculture-de-conservation.com/sites/agriculture-de-conservation.com/IMG/pdf/pdfsam_potentiel_redoxtcs99_light.pdf
- ↑ K. R. REDDY and W. H. PATKIC'K. JK, Effect of alternate aerobic and anaerobic conditions on redox potential, organic matter decomposition and nitrgoen loss in a flooded soil, Biol. Biochem. Vol 7, pp 87-94, Pergamon Press 1995
- ↑ 5.0 5.1 Kralova, M., Masscheleyn, P.H., Lindau, C.W. et al. Production of dinitrogen and nitrous oxide in soil suspensions as affected by redox potential. Water Air Soil Pollut 61, 37-45 (1992). https://doi.org/10.1007/BF00478364